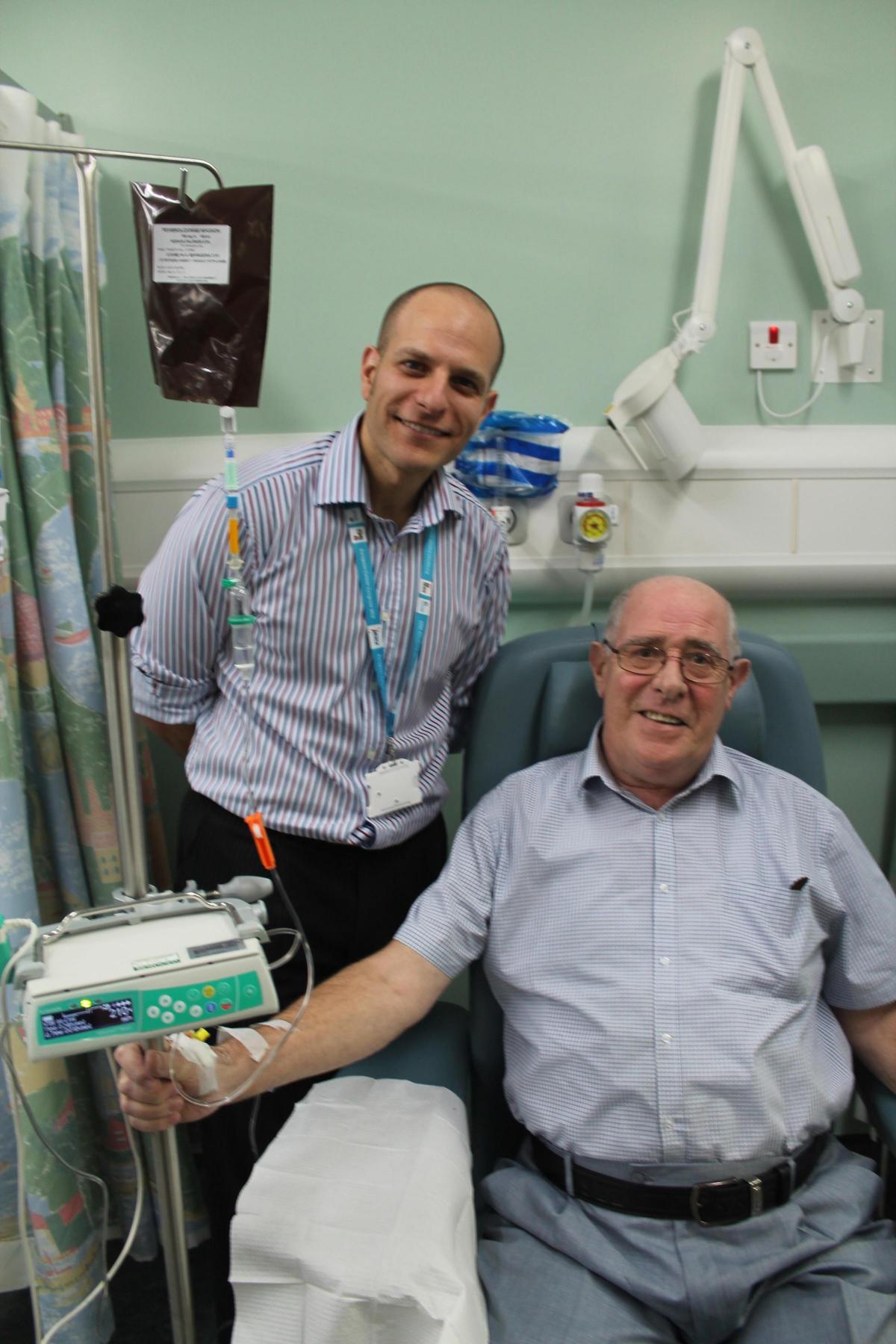
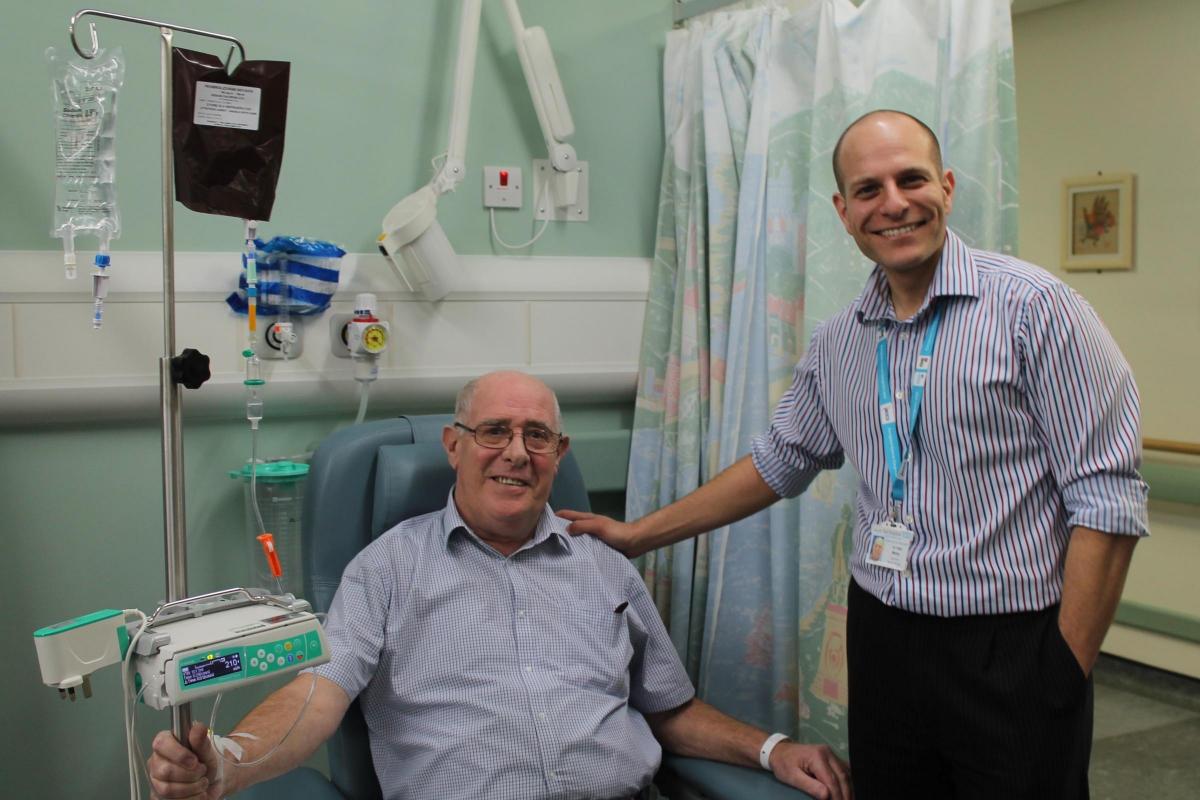
A TEESSIDE man has become the first NHS patient in the country to be given a new immunotherapy drug for the treatment of lung cancer.
Ken Hall, 69, of Eston, Middlesbrough, was told he may have less than a year to live when a persistent cough led to a lung cancer diagnosis in April 2014.
But the retired British Steel engineer responded so well to chemotherapy treatment that his consultant, Dr Talal Mansy, made a special request to use an immunotherapy drug called Pembrolizumab through the Early Access to Medicines Scheme.
“I’m really excited about it,” said Mr Hall.
“The chemotherapy had limited success but I’m hoping the effects of this will last longer.
“I had two lots of chemotherapy and I was okay for nine months but then it started to grow again. I knew I wasn’t right because I lost my appetite and was short of breath.
“Dr Mansy said he would put me in for more chemotherapy but then he mentioned this new treatment. He made some enquiries and was really keen to try it. He thought it would work so I agreed.”
Immunotherapy is designed to stimulate the body’s immune system to fight cancer cells and is less toxic than chemotherapy.
Dr Mansy said: “Cancer switches off the body’s immune system, stopping the immune system from fighting the cancer. Immunotherapy is designed to switch the immune system back on, which allows the body’s immune system to then fight the cance.
“It’s very exciting for my patient and for James Cook as we are the first centre in the UK to get access to this drug for lung cancer patients as part of the Early Access to Medicines Scheme. Previously it has only been available for lung cancer patients in clinical trials or privately.”
Mr Hall's wife, Jean, said: “Dr Mansy and his team worked very hard to make it happen and we are very grateful.”
The treatment is long-term and will be given intravenously for 30 minutes every three weeks, but it is only suitable for patients meeting strict criteria and side effects have to be carefully monitored.
The Early Access to Medicines Scheme aims to give patients with life threatening conditions access to medicines that do not yet have a marketing authorisation when there is a clear unmet medical need.
Under the scheme, the Medicines and Healthcare products Regulatory Agency give a scientific opinion on the benefit and risk balance of the medicine, based on the data available.



Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules hereLast Updated:
Report this comment Cancel